Akari Therapeutics’ Nomacopan Demonstrates Positive Early Safety and Efficacy in Phase I/II Clinical Study in Moderate-to-Severe Atopic Keraconjunctivitis (AKC)
- Part A of a safety and efficacy study successfully completed with nomacopan eye drops which were well tolerated with no serious adverse events.
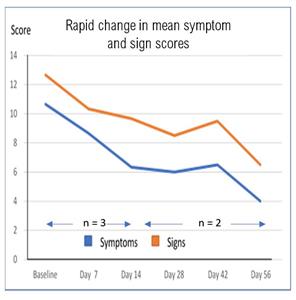
- Improvement in mean composite clinical efficacy score of 35% by Day 14 and 55% by Day 56. All patients were on maximal dosage of cyclosporin, the standard of care, for at least three months prior to treatment.
- Improvement in the composite clinical score was seen in signs and symptoms reflecting the potential for both reduced corneal damage and improved patient comfort.
- Part B randomized placebo-controlled portion of the study currently recruiting with data read out planned fourth quarter 2019.
NEW YORK and LONDON,
|
|||||
AKC is a serious corneal and eye surface disease which frequently progresses to visual impairment. Currently, there are no approved drugs and current standard of care is based on topical and systemic immunosuppression. The current treatments are considered sub-optimal because they frequently fail to prevent progression of the disease and the topical drops are often associated with a high incidence of pain and irritation.
“The results from the first three patients included in Part A exceeded our expectations. The rapid response and the overall reduction of 55% in the composite clinical score by Day 56 despite the patients starting treatment while on long term maximal cyclosporin, is very encouraging in AKC which has no approved therapy and limited effective treatment options,” said Clive Richardson, Interim Chief Executive Officer of Akari Therapeutics. “AKC is often associated with severe dry eye disease (DED) and as such may be a potential gateway into the broader and significant multi-billion dry eye disease market”.
This early data in AKC further supports the therapeutic efficacy of nomacopan, a dual acting complement C5 and LTB4 inhibitor, observed in initial clinical data for the treatment of bullous pemphigoid and thrombotic microangiopathy after haematopoietic stem cell transplantation which, like AKC, have no approved treatments.
In Part A of the Phase I/II study, three patients were treated with twice daily nomacopan eye drops in addition to standard of care for up to 56 days in order to establish the safety and tolerability of the drops in preparation for Part B, a randomized, double-masked placebo-controlled comparison in 16 patients. Of the three patients enrolled in the study, two completed 56 days of treatment and one completed 14 days and then withdrew for reasons unrelated to the study treatment. All patients, who were on the moderate/severe end of the AKC spectrum, had been on maximal topical cyclosporin, the standard of care, for at least three months prior to entry and continued on it during the trial. In the event of further disease progression, the next incremental step would normally have been systemic immunosuppression.
The drops were found to be comfortable and well-tolerated throughout the trial for all three patients. There were no serious adverse events (SAE) reported. On that basis, the independent safety committee has now given permission for the trial to proceed to Part B and recruitment has commenced.
The secondary objective of the study was to determine efficacy, assessed by a standard composite scoring system [Akpek E.K. et al. Ophthalmology 2004 (III,3)] consisting of five symptoms which were patient reported, and six signs of ocular damage which were graded by the clinician on a direct slit-lamp examination of the eye. Each sign or symptom was graded 0 to 3, where 0 = normal or absent and 3 is the most severe, such that with 11 measures the overall maximum severity score was 33.
There was an overall improvement in clinical score of 55% composed of an improvement in symptoms of 62% and signs of 52% by Day 56. Symptoms consist of subjective occurrences such as discomfort and itching. Signs are objective manifestations of disease, such as conjunctival redness, growth of new blood vessels into the cornea and microscopic damage to the corneal surface (punctate keratitis).
In addition, post-instillation comfort was reported by patients as excellent with high levels of acceptance of eye drops, which were described as comfortable and refreshing.
Mr Sajjad Ahmad, consultant ophthalmic surgeon at
About Akari Therapeutics
Akari is a biopharmaceutical company focused on developing inhibitors of acute and chronic inflammation, specifically for the treatment of rare and orphan diseases, in particular those where the complement (C5) or leukotriene (LTB4) systems, or both complement and leukotrienes together, play a primary role in disease progression. Akari's lead drug candidate, nomacopan (formerly known as Coversin), is a C5 complement inhibitor that also independently and specifically inhibits leukotriene B4 (LTB4) activity. Nomacopan is currently being clinically evaluated in four indications: bullous pemphigoid (BP), atopic keratoconjunctivitis (AKC), thrombotic microangiopathy (TMA), and paroxysmal nocturnal hemoglobinuria (PNH). Akari believes that the dual action of nomacopan on both C5 and LTB4 may be beneficial in AKC and BP. Akari is also developing other tick derived proteins, including longer acting versions.
Cautionary Note Regarding Forward-Looking Statements
Certain statements in this press release constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control. Such risks and uncertainties for our company include, but are not limited to: needs for additional capital to fund our operations, our ability to continue as a going concern; uncertainties of cash flows and inability to meet working capital needs; an inability or delay in obtaining required regulatory approvals for nomacopan and any other product candidates, which may result in unexpected cost expenditures; our ability to obtain orphan drug designation in additional indications; risks inherent in drug development in general; uncertainties in obtaining successful clinical results for nomacopan and any other product candidates and unexpected costs that may result therefrom; difficulties enrolling patients in our clinical trials; failure to realize any value of nomacopan and any other product candidates developed and being developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; inability to develop new product candidates and support existing product candidates; the approval by the FDA and EMA and any other similar foreign regulatory authorities of other competing or superior products brought to market; risks resulting from unforeseen side effects; risk that the market for nomacopan may not be as large as expected; risks associated with the departure of our former Chief Executive Officers and other executive officers; risks associated with the SEC investigation; inability to obtain, maintain and enforce patents and other intellectual property rights or the unexpected costs associated with such enforcement or litigation; inability to obtain and maintain commercial manufacturing arrangements with third party manufacturers or establish commercial scale manufacturing capabilities; the inability to timely source adequate supply of our active pharmaceutical ingredients from third party manufacturers on whom the company depends; unexpected cost increases and pricing pressures and risks and other risk factors detailed in our public filings with the
For more information
Investor Contact:
Peter Vozzo
Westwicke Partners
(443) 213-0505
peter.vozzo@westwicke.com
Media Contact:
Sukaina Virji / Nicholas Brown / Lizzie Seeley
Consilium Strategic Communications
+44 (0)20 3709 5700
Akari@consilium-comms.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/437749b1-3857-4c16-a1cc-8efaf4aa2cdb
Source: Akari Therapeutics Plc