UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of: May 2023
Commission file number: 001-36288
Akari Therapeutics, Plc
(Translation of registrant’s name into English)
75/76 Wimpole Street
London W1G 9RT
United Kingdom
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F x Form 40-F ¨
On May 22, 2023, Akari Therapeutics, Plc, a public company with limited liability incorporated under the laws of England and Wales (the “Company”), posted an updated investor presentation on its website. A copy of the Company’s presentation is furnished as Exhibit 99.1 to this Report on Form 6-K and is incorporated herein by reference.
The information in slides 24 and 26 of such presentation is hereby incorporated by reference into all effective registration statements filed by the Company under the Securities Act of 1933, as amended.
| Exhibit No. | |
| 99.1 | Slide Presentation. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Akari Therapeutics, Plc | ||
| (Registrant) | ||
| By: | /s/ Rachelle Jacques | |
|
Name: Title: |
Rachelle Jacques President and Chief Executive Officer | |
Date: May 22, 2023
Exhibit 99.1

Akari Therapeutics May 2023

2 Certain statements in this presentation constitute “forward - looking” statements within the meaning of Section 27A of the Securit ies Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward - looking statements reflect the current views of Akari Therapeutics, Plc (the “Company”, “we”,. “our” and “us”) and its p lans, intentions, expectations, strategies and prospects, which are based on the information currently available to it and on assumptions it has made. Although we believe that our plans, intentions, expecta tio ns, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained o r a chieved. Furthermore, actual results may differ materially from those described in the forward - looking statements and will be affected by a variety of risks and factors that are beyond our control. Such risks and un certainties for our company include, but are not limited to: needs for additional capital to fund our operations; our ability to continue as a going concern; uncertainties of cash flows and inability to meet working ca pit al needs; an inability or delay in obtaining required regulatory approvals for nomacopan (Coversin) and any other product candidates that may result in unexpected cost expenditures; our ability to successfully deve lop nomacopan as a treatment for COVID - 19 related pneumonia and to successfully commercialize any product in that indication; our ability to obtain orphan drug designation in additional indications; risks inh erent in drug development in general, and risks specific to the development of potential treatments for COVID - 19 - related illnesses; uncertainties in obtaining successful clinical results for nomacopan and any other pr oduct candidates and unexpected costs that may result from difficulties enrolling patients in our clinical trials; failure to realize any value of nomacopan and any other product candidates developed or bein g d eveloped in light of inherent risks and difficulties involved in successfully bringing product candidates to market; inability to develop new product candidates and support existing product candidates; the approv al by the FDA and EMA an any other similar foreign regulatory authorities of other competing or superior products brought to market; risks resulting from unforeseen side effects; risk that the market opportun ity for nomacopan may not be as large as expected; risks associated with a resurgence of the COVID - 19 pandemic; inability to obtain, maintain and enforce patents another intellectual property rights or the unexpected costs associated with such enforcement or litigation; inability to obtain and maintain commercial manufacturing arrangements with third party manufacturers or establish commercial scale manufacturing capabilities ; t he inability to timely source adequate supply of our active pharmaceutical ingredients from third - party manufacturers on whom the company depends; unexpected cost increases and pricing pressures and risk s and other risk factors detailed in our public filings with the U.S. Securities and Exchange Commission; including our most recently filed Annual Report on Form 20 - F filed with the SEC. The statements made in this presentation speak only as of the date stated herein, and subsequent events and developments may cau se our expectations and beliefs to change. Unless otherwise required by applicable securities law, we do not intend, nor do we undertake any obligation to update or revise any forward - looking statemen ts contained in this presentation to reflect subsequent information, events, results or circumstances or otherwise. While we may elect to update these forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law. Unless otherwise indicated, information contained in this presentation concerning our industry, competitive position and the mar kets in which we operate is based on information from independent industry and research organizations, other third - party sources and management estimates. Management estimates are derived from publicly avail able information released by third - party sources, as well as data from our internal research, and are based on assumptions made by us upon reviewing such data, and our experience in, and knowledge of, such ind ust ry and markets, which we believe to be reasonable, but we have not independently verified the accuracy of this information. Any industry forecasts are based on data (including third - party data), models and experience of various professionals and are based on various assumptions, all of which are subject to change without notice. In addition, projections, assumptions and estimates of the future performa nce of the industry in which we operate and our future performance are necessarily subject to uncertainty and risk due to a variety of factors, including those described in “Cautionary Note Regarding Forward - Looking Sta tements.” These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use shou ld not be construed as an endorsement of such products. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale o f t hese securities in any state or other jurisdiction Forward - Looking Statements

Akari Overview Nomacopan is a unique asset inhibiting 2 co - dependent, proinflammatory targets: complement C5 and leukotriene B4 (LTB4) 3 Potential for use in several diseases; commercial flexibility due to multiple routes of administration (subcutaneous, topical, intravitreal, IV) 1. Novel C5+LTB4 inhibitor 2. Broad potential Extensive clinical and safety data from multiple clinical trials 3. Robust clinical dataset 2 IN 1 Phase 3 clinical trial in pediatric hematopoietic stem cell transplant - related thrombotic microangiopathy (HSCT - TMA); no approved therapies and ~80% mortality; FDA Orphan, Fast Track, and Rare Pediatric Disease designations; potential for adult follow - on indication 4. HSCT - TMA Phase 3 5. GA Pre - Clinical Pre - clinical program investigating PAS - nomacopan in geographic atrophy (GA) with target dose interval of 3 months or longer without increased risk of choroidal neovascularization (CNV)

4 Discovered nomacopan Volution Torsten Hombeck, PhD Chief Financial Officer Miles Nunn, DPhil Chief Scientific Officer Rachelle Jacques President & CEO Melissa Bradford - Klug Chief Operating Officer John Neylan , MD Chief Medical Officer Leadership Team Board member Board member

• Inhibits complement C5 activation similarly to an on - market complement inhibitor ablating effects of terminal complement activation • Sequesters LTB4, disrupting activation and recruitment of immune modulating cells responsible for damaging inflammation 5 High resolution structure of nomacopan (blue) bound to human complement C5 1 High resolution structure of nomacopan capture of LTB4 (yellow) )2 Nomacopan Is a Dual Action Recombinant Protein Discovered In Ticks References 1. Jore MM, Johnson S, Sheppard D, et al. Structural basis for therapeutic inhibition of complement C5. Nat Struct Mol Biol. 2016;23(5):378 - 386. 2. Roversi P, Ryffel B, Togbe D, et al. Bifunctional lipocalin ameliorates murine immune complex - induced acute lung injury. J Biol Chem. 2013;288(26):18789 - 18802. T icks secrete immunomodulatory proteins that help them control host responses (inflammation, pain, itch and blood flow). These are the same responses that may be out of control in certain human autoimmune and inflammatory conditions. Novel, bispecific nomacopan is a recombinant protein derived from nature via discovery in ticks

• Bispecific mechanism prevents two separate, but related, tissue - damaging effects • Opsonization (antibody binding) and role of complement in clearance of immune complexes that are needed for healthy immune response remain intact • LTB4 is a key mediator of inflammation that: o Is independently activated from complement o Can amplify the effects of complement activation o Has independent potent inflammatory actions 1. Nunn MA, Sharma A, Paesen GC et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata . J Immunol. 2005; 174:2084 - 2091 C5a, LTB4 and MAC act jointly on neutrophils, macrophages and other cell types that can cause inflammation and damage Cell activation, lysis and autoimmunity nomacopan 1 2 Complement Leukotriene Anaphylatoxins CONFIDENTIAL Nomacopan does not impair the anti - inflammatory action of the leukotriene pathway Nomacopan Inhibits Two Pathways That Can Cause Damaging Inflammation, While Preserving Important Immune Functions 6

LTB4 & C5 Are Separate Pathways But In Vivo Data Point to Signalling Interplay That Leads to Damaging Inflammation References 1. Sadik CD, et al. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and Fc γ R signaling. Proc Natl Acad Sci U S A . 2012;109(46):E3177 - E3185. 2. Sadik CD et al. Lipid - cytokine - chemokine cascades o rchestrate leukocyte recruitment in inflammation . J Leuk Biol. 2012; 91(2 :207 - 215. ; 3. Roversi P, et al. Bifunctional lipocalin ameliorates murine immune complex - induced acute lung injury. J Biol Chem . 2013;288(26):18789 - 18802 • Neutrophils infiltrate joints by way of multiple chemoattractant receptors, including LTB4 (BLT1) and chemokine receptors • In the joint, neutrophils perpetuate their own recruitment by releasing LTB4 and IL - 1 β • Complement C5aR activation of neutrophils is required for LTB4 release and early neutrophil recruitment into the joint 7 • C5 and LTB4 contribute equally to this model of IC - ALI • C5a receptor signaling regulates Fc receptors promoting inflammation • Activated alveolar macrophages produce proteases, cytokines & LTB4 • C5a and LTB4 receptor activation upregulate adhesion molecules, recruit & degranulate neutrophils releasing super - oxides, causing further inflammation and microvascular damage LTB4 initiates cytokine and chemokine cascade in the joint Inflammation at the alveolar surface In vivo study of autoantibody - induced inflammatory arthritis 1, 2 In vivo study of immune complex - induced acute lung injury (IC - ALI) 3

Geographic atrophy (GA) Long - acting PAS - nomacopan/ intravitreal injection FDA Orphan, Fast Track, and Rare Pediatric Disease designations Pediatric hematopoietic stem cell transplant – related thrombotic microangiopathy (HSCT - TMA) Nomacopan/ subcutaneous Near - Term Potential, Promising Pre - Clinical Program 8 FDA Orphan Drug, Fast Track and Rare Pediatric Disease designations Designations Indication Candidate/ Formulation Phase 3 Parts A & B Phase 1 Pre - Clinical Adult hematopoietic stem cell transplant – related thrombotic microangiopathy (HSCT - TMA) Nomacopan/ subcutaneous Phase 2 IND filing expected in 1H 2024 Study enrollment expected to open in 2024 Advancing to pivotal Part B in >2 years age cohorts, on track to begin enrollment in Q4. Part A remains open for enrollment in <2 years age cohort FDA Orphan Drug Designation

• In addition to current areas of focus, Akari has conducted clinical research in several other areas, including Phase 2/3 clin ica l trials of subcutaneous nomacopan for treatment of bullous pemphigoid (BP) and paroxysmal nocturnal hemoglobinuria (PNH) • This research set a solid foundation for the current Phase 3 clinical trial in pediatric HSCT - TMA Previous Areas of Clinical Development, Including PNH and BP, Support Current Development Pathways • In clinical studies of nomacopan in BP, 7 of 9 patients responded to nomacopan 1 o 3 showed >80% reduction in BPDAI by day 42 (BP disease activity) All prior treatment, including steroids, withdrawn ~ one week prior to initiation of treatment with nomacopan. Lesional mometasone was administered to Day 21. • In a Phase 3 study in PNH, 100% of untreated patients were transfusion dependent while 0% of nomacopan patients were transfusion dependent o >32 patient years of nomacopan exposure in PNH in 19 patients Proportion of PNH patients who were transfusion independent following entry to trial 1. Sadik CD, et al. Evaluation of nomacopan for treatment of bullous pemphigoid a phase 2a non0randomized controlled trial. JAMA Dermatol. 2022; 158: 641 - 649 N=4 N=5 9

10 THROMBOTIC MICROANGIOPATHIES (TMAs)

11 • TMA following a stem cell transplant procedure is a rare but serious complication of HSCT that appears to involve complement activation, inflammation, tissue hypoxia and blood clots, leading to progressive organ damage and death • Mortality in patients who develop severe transplant - related TMA is 80% (across both adults and children) 1 • Currently, there are no approved treatment options in the U.S. or Europe Nomacopan May Be the First Treatment for Pediatric HSCT - TMA, a Condition with Mortality Up to 80% 1. Efficacy Efficacy of complement C5 inhibition by eculizumab is supported in HSCT - TMA 2 and atypical hemolytic uremic syndrome ( aHUS ) 3,4 , another TMA; efficacy of nomacopan C5 inhibition supported by clinical PNH research 5 2. Dosing Nomacopan clinical trials are establishing a simple, fixed dose for each age category; rapid offset of action allows complement re - activation if/when needed 3. GVHD Graft versus host disease (GVHD) is commonly present in patients with severe HSCT - TMA 6; LTB4 is often elevated in patients with GVHD and inhibition of LTB4 may slow GVHD progression 7 1. Rosenthal J. Hematopoietic cell transplantation - associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. J Blood Med. 2016;7:181 - 186. 2. Jodele S, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation - associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518 - 525. 3. Licht C, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2 - year extensions of phase 2 studies. Kidney Int. 2015;87(5):1061 - 1073. 4. Greenbaum LA, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701 - 711. Kidney Int. 2016;90(3):709 5. Schols S, Nunn MA, Mackie I et al. Succesful treatment of a PNH patient non - responsive to eculizumab with novel complement C5 inhibitor covers (nomacopan). Br J Hematol . 2020; 188: 332 - 340. 6. Jodele S, et al. Complement blockade for TA - TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood . 2020;135(13):1049 - 1057. 7. Takatsuka H, et al. Predicting the severity of intestinal graft - versus - host disease from leukotriene B4 levels after bone marrow transplantation. Bone Marrow Transplant. 2000;26(12):1313 - 1316.

References • Health Resources and Services Administration (HRSA), 2020 • Jodele S, et al. Diagnostic and risk criteria for HSCT - associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645 - 653. • Jodele S, et al. Complement blockade for TA - TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049 - 1057. 12 Pediatric & Adult HSCT - TMA U.S. Market Opportunity Adult population >10X pediatric ~ 4,600 ~ 400 ~ 3,400 0 2,500 5,000 7,500 10,000 Moderate to severe not complement mediated Moderate to severe complement mediated Mild HSCT - TMA U.S. Population (incidence in adults and children in the U.S. 2022) ~22,000 HSCTs occur annually in the U.S. ~8,400 (38%) lead to TMAs ~3,400 ~3,400 TMAs are moderate to severe complement - mediated o 200 - 300 pediatrics o 3,100 - 3,200 adult PATIENT POPULATION IN THOUSANDS

Nomacopan in HSCT - TMA Recent Progress 1 Acceleration into pivotal, registrational Phase 3 Part B 13 Based on FDA guidance, moving forward to design and planning for pivotal Part B of Phase 3 clinical trial in pediatric HSCT - TMA patients 2 years to <18 years of age 2 Addition of new pipeline program in adult HSCT - TMA 3 Phase 3 Part A to remain open for youngest pediatric patients Initiating new program for indication in adults with HSCT - TMA, which will include a study supportive of both adult and pediatric regulatory pathways; adult HSCT - TMA population is >10 times pediatric Enrollment for youngest group (0.5 to <2 years) in Part A is not complete and study will stay open for these patients while pivotal Part B study in the older pediatric patients advances; Part A data readout upon completion
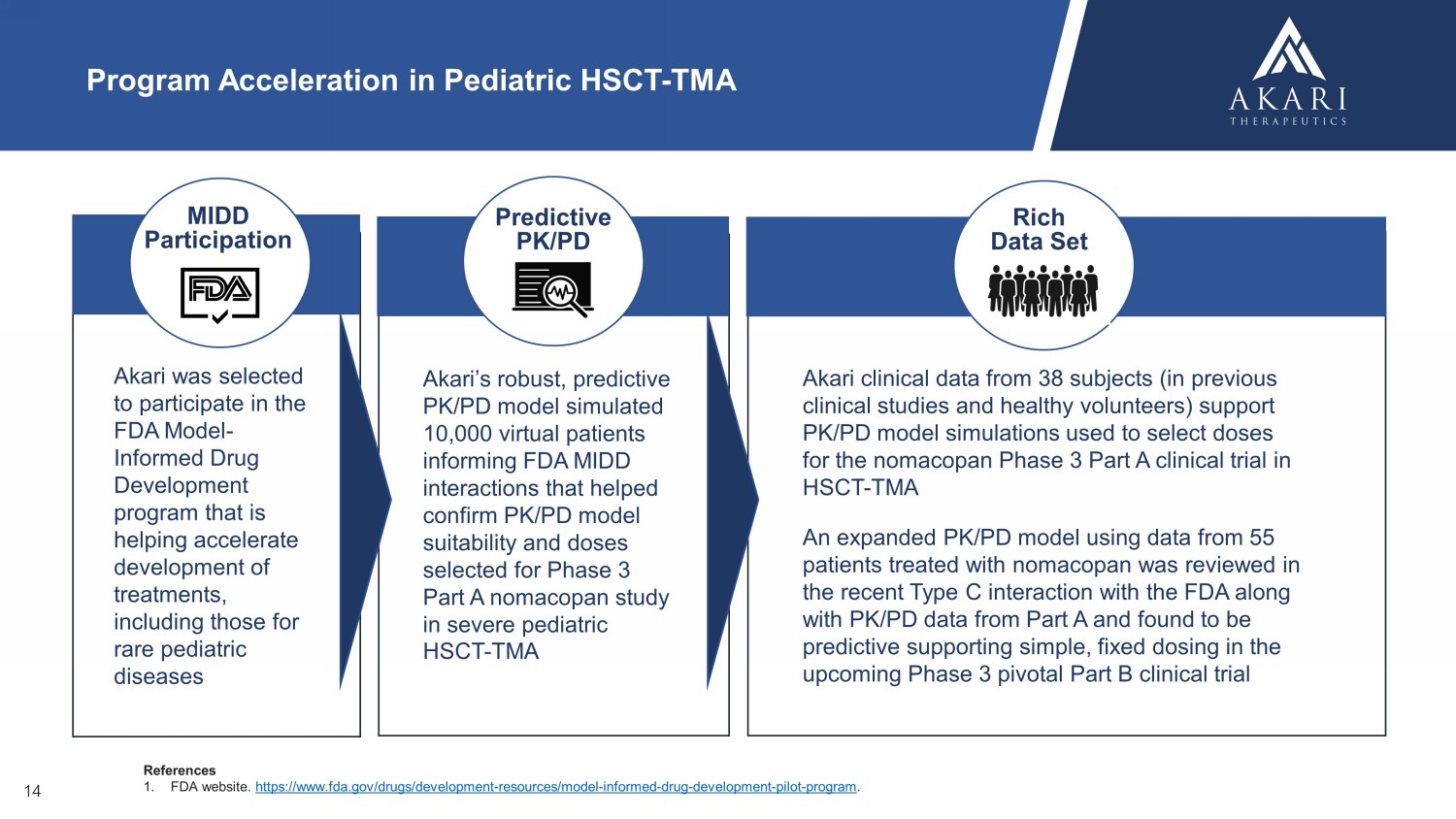
References 1. FDA website. https://www.fda.gov/drugs/development - resources/model - informed - drug - development - pilot - program . Akari was selected to participate in the FDA Model - Informed Drug Development program that is helping accelerate development of treatments, including those for rare pediatric diseases Predictive PK/PD Model Akari’s robust, predictive PK/PD model simulated 10,000 virtual patients informing FDA MIDD interactions that helped confirm PK/PD model suitability and doses selected for Phase 3 Part A nomacopan study in severe pediatric HSCT - TMA MIDD Participation Akari clinical data from 38 subjects (in previous clinical studies and healthy volunteers) support PK/PD model simulations used to select doses for the nomacopan Phase 3 Part A clinical trial in HSCT - TMA Program Acceleration in Pediatric HSCT - TMA Rich Data Set 14 An expanded PK/PD model using data from 55 patients treated with nomacopan was reviewed in the recent Type C interaction with the FDA along with PK/PD data from Part A and found to be predictive supporting simple, fixed dosing in the upcoming Phase 3 pivotal Part B clinical trial

15 Clinical Trial Patient Case Study Presented As Late - Breaker at Transplantation and Cellular Therapy Tandem Meetings A patient with severe pediatric HSCT - TMA, which typically involves multi - organ failure and other acute consequences, was discharged home from the hospital following treatment with nomacopan • 6 - year - old male received a cord blood HSCT for relapsed refractory acute myelogenous leukemia (AML) • Post - transplant acute gut graft - versus - host disease (GVHD) • TMA at d ay +66 post - transplant • T reatment with a single - age, weight - based ablating dose of nomacopan day +74 followed by maintenance dosing for 21 days • After a 3 - day break in treatment for encephalopathy unrelated to nomacopan, treatment continued for a further 46 days until the end of the study with correction of the patient’s urine protein creatinine ratio for ≥28 days • Gut pathology and thrombocytopenia resolved • No adverse events related to nomacopan Clinical Response to Nomacopan in the Pediatric HSCT - TMA Setting presented Feb. 16, 2023, at the Transplantation & Cellular Therapy Tandem Meetings. P oster available http:// investor.akaritx.com /news - and - events/presentations Break in nomacopan treatment for 3 days due to SAE (determined unrelated to nomacopan)

16 GEOGRAPHIC ATROPHY (GA)

17 Geographic Atrophy (GA) • Geographic atrophy (GA) manifests as a chronic progressive degeneration of the macula, which occurs during late - stage dry age - related macular degeneration (dAMD) and can lead to irreversible vision loss • Approximately 5 million people worldwide are affected, 1,2 with nearly 1 million in the U.S. 3 • One treatment was approved by the FDA in 2023 References 1. Wong WL, et al. Global prevalence of age - related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta - analysis. Lancet Glob Health. 2014;2(2):e106 - e116. 2. Rudnicka AR, et al. Age and gender variations in age - related macular degeneration prevalence in populations of European ancestry: a meta - analysis. Ophthalmology. 2012;119(3):571 - 580. 3. Friedman DS, et al. Prevalence of age - related macular degeneration in the United States [published correction appears in Arch Ophthalmol . 2011 Sep;129(9):1188]. Arch Ophthalmol . 2004;122(4):564 - 572.

Complement - Only Inhibitors Have Demonstrated Promising Efficacy in GA, Yet Significant Treatment Burdens Exist 18 Stage Drug MOA Injections/year with no CNV Reduction GA growth (mm 2 ) vs sham 1, 2 Dose interval avacincaptad pegol ( Zimura ® ) Incidence of CNV 2,3 pegcetacoplan ( Syfovere TM ) PAS - nomacopan / Akari anti - C3 PEGylated peptide, IVT anti - C3 PEGylated aptamer, IVT anti - C5 PASylated small protein, IVT FDA approved Feb 2023 PDUFA Aug 2023 Pre - clinical 25 to 60 days Monthly 3+ months EM: 17% EOM: 14% (pooled 12 - month data DERBY & OAKS) EM: 17.3% (12 - month data GATHER2) EM: 12% EOM: 7% SHAM: 3.1% (at 24 months) EM: 7.2% SHAM: 3.6% (at 12 months) Address via LTB4 inhibition/ target equivalent to sham Injections/year with CNV 6 - 14 injections 12 injections 4 injections or fewer 12 - 22 injections (6 – 8 anti - VEGF) 18 - 20 injections N/A References : 1. Presentation DERBY and OAKS trial results Oct 11 2021, American Society of Retina Specialists 2021, San Antonio, Texas; 2. Iveric GATHER - 2 press release 6 Sept 2022 - table in Supplement showing GATHER - 1 and GATHER - 2 results at 12 - months; 3. Apellis DERBY and OAKS 24 month data press release August 24 2022; 4. Medscape article on 24 - month data presentation at AAO 2022 With Approval Pending, Pegcetacoplan Shows Mixed Results for Treating Geographic Atrophy https://www.medscape.com/viewarticle/981813#vp_2 5. McClard CK, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII). BMJ Open Ophthalmol . 2021;6(1):e000669. TBD • In clinical trials discontinuation for an approved complement - only inhibitor for GA treatment reported up to 20% 4 • For anti - VEGF CNV treatments, up to 1/3 of patients may discontinue/ not adhere 5

PAS - Nomacopan May Provide 3 Key Benefits: Complement Inhibition, Fewer Doses and LTB4 Inhibition to Address CNV Risk 19 References 1. Liao DS, et al., Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age - related macular degeneration - a randomised phase 2 trial. Opthalmology 2019; 127: 586 - 195. 2. Jaffe GJ, et al., C5 inhibitor avacincaptad peg for geographic atrophy due to age - related macular degeneration - a randomised pivotal phase 2/3 trial. Ophthalmology 2021; 128: 576 - 586. 3. McClard CK, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a patient - reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol . 2021;6(1):e000669. 4. Sasaki F, et al., Leukotriene B4 promotes neovascularisation and macrophage recruitment in murine wet - type AMD models. JCI Insight 2018; 3: e96902. 1. Efficacy Efficacy of complement C3 and C5 inhibition slowing progression of GA lesions is well understood 1,2 2. Frequency of Intravitreal Injections Frequent needle injections into the back of the eye, a source of fear, discomfort and disruption for patients 3 ; p otential for 4 or fewer injections with PAS - nomacopan each year 3. Safety LTB4 inhibition may prevent VEGF - A overexpression, a key driver of sight - threatening CNV, 4 a safety risk (treated with VEGF inhibitors) associated with approved and late - stage complement - only inhibitors New PAS - nomacopan construct composition of matter patent filed Dec. 2022; if granted provides patent protection to 2042

Long - Acting PAS - Nomacopan Has Potential to Extend Intravitreal Dose Interval to 3 Months or Longer 1 10 100 1,000 10,000 0 20 40 60 80 100 120 PAS - nom concentration vitreous ( µg/mL) Time (days) 0.05mL PAS600-nomacopan (20 mg/mL) 0.05mL PAS600-nomacopan (60 mg/mL) Long - Acting PAS - Nomacopan Has Potential for 4 or Fewer Injections Into the Eye Per Year • PK/PD data show PAS - nomacopan has extended half - life in the eye after intravitreal injection (7.4 to 8.4 days), suggesting the dose interval may be 3 months or longer 1 Reference: 1. Weston - Davies, W., et al. Development of long - acting PAS - nomacopan for treatment of GA and other retinal diseases. Poster presen tation ARVO, 2022. 20 OBSERVED DATA IN RABBITS Predicted concentration 0.1mL PAS - nomacopan (60mg/mL) 50μL PAS - nomacopan 20mg/mL 50μL PAS - nomacopan 60mg/mL Source: PAS - nomacopan data generated from internal studies Retinal Bioavailability Retina 0 5 10 15 20 25 30 35 40 45 50 0 7 14 21 28 % OF VITREOUS CONCENTRATION (MEAN ± SEM) DAYS POST INJECTION Aflibercept PAS-nomacopan

• CNV is an overdevelopment of blood vessels in the retina 2 • New blood vessels are leaky , fluid from blood/red blood cells enter the retina 2 • Fluid can distort/damage the retina , including photoreceptors 2 Neovascularization, fluid, retinal/macular distortion 2 CNV starts with inflammation in the choroid and retinal pigment epithelium (RPE) • The choroid is part of the vascular layer of the eye 1 • The RPE , adjacent to the choroid, is constantly exposed to high levels of metabolic and oxidative stress 1 choroid retinal pigment epithelium photoreceptor cells retina (macular) LTB4 Inhibition May Prevent Choroidal Neovascularization 1 • The RPEs ability to cope with stress decreases with age and the subsequent inflammation damages the RPE and photoreceptors 2 • Damaged RPE releases leukotrienes, including LTB4 2,3 LTB4 activation can lead to over expression of VEGF - A 2 • In a pre - clinical model of laser - induced CNV LTB4 recruited inflammatory immune cells into the retina 3 • M2 macrophages were attracted and activated via LTB4 receptors leading to production of vascular endothelial growth factor – A (VEGF - A) 3 3 Normal expression of VEGF - A is healthy • VEGF - A is one of the key factors responsible for endothelial cell proliferation and migration • Endothelial cells form the inner layer of blood vessels and play a key role in function, including exchanges between blood vessels and surrounding tissues 4 Overexpression of VEGF - A drives choroidal neovascularization LTB4 can upregulate the production of VEGF - A, a key driver of CNV. 3 CNV is responsible for 90% of severe vision loss in AMD patients and eyes with CNV experience greater vision loss than GA only VEGF - A protein References: 1. Hejtmancik JF, Nickerson JM. Overview of the Visual System. Prog Mol Biol Transl Sci. 2015;134:1 - 4. . 2. Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol . 2004;137(3):496 - 503. 3. Sasaki F, Koga T, Ohba M, et al. Leukotriene B4 promotes neovascularization and macrophage recruitment in murine wet - type AMD models. JCI Insight 2018;3(18):e96902. Published 2018 Sep 20. 4. Guyer D.R., et al. Subfoveal choroidal neovascular membranes in age - related macular degeneration. Visual prognosis in eyes with relatively good initial visu al acuity. Arch Ophthalmol . 1986;104:702 – 705 5. Wong T.Y., et al. The natural history and prognosis of neovascular age - related macular degeneration: A systematic review of the literature and meta - analysis. Ophthalmology 2008;115:116 – 126. 21

PAS - Nomacopan Decreased VEGF Levels As Effectively As An Anti - VEGF Antibody In a Pre - Clinical Model V E G F ( p g / m l ) S a l i n e n o m a P A S - n o m a a n t i - V E G F H e a l t h y c t 0 100 200 300 p=0.044 p=0.048 Effect of PAS - nomacopan on VEGF levels in a standard pre - clinical model of severe uveitis 22 In a pre - clinical model of severe uveitis, long - acting PAS - nomacopan (single IVI) decreased VEGF levels (VEGF - A is a key driver of CNV) as effectively as anti - VEGF antibody treatment 1,2 LTB4 promotes laser induced CNV in a pre - clinical model of wet age related macular degeneration 3 Intravitreal VEGF concentration ( pg /ml) Nomacopan Saline Anti - VEGF Healthy control PAS - Nomacopan References 1. Eskandarpour M, et al., Leukotriene B4 and its receptor in experimental autoimmune uveitis and in human retinal tissues – clinical severity and LTB4 dependence of retinal Th17 cells. Am J Pathol . 2021; 191:320 - 334 2. Eskandapour M, et al., Immune mediated retinal vasculitis in posterior uveitis and experimental models: the leukotriene (LT)B4 - VEGF axis. Cells 2021; 10:396 3. Sasaki F, et al., Leukotriene B4 promotes neovascularization and macrophage recruitment inn murine wet - type AMD models. JCI Insight 2018; 3:e96902

23 NEXT STEPS

Next Steps 24 • First - in - human/ initiation of clinical trials 2H 2024 • IND filing expected 1H 2024 • IND - enabling studies underway 2023 2024 • Initiate clinical supply PAS - Nomacopan in GA Nomacopan in HSCT - TMA • Enrollment expected to begin in Phase 3 Part B clinical trial in pediatric HSCT - TMA • Initiation of clinical trial in adult HSCT - TMA

25 FINANCIAL UPDATE

Financial Update 26 • Ticker: AKTX (NASDAQ) • 101.1M ADS outstanding • Cash of $13.2M as of December 31, 2022 • Additional gross proceeds of $4M raised in a registered direct offering in March 2023 • Estimated cash runway into Q4 2023

27 THANK YOU
