UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16
under the Securities Exchange Act of 1934
For the month of: February 2023
Commission file number: 001-36288
Akari Therapeutics, Plc
(Translation of registrant’s name into English)
75/76 Wimpole Street
London W1G 9RT
United Kingdom
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F x Form 40-F ¨
On February 15, 2023, Akari Therapeutics, Plc, a public company with limited liability incorporated under the laws of England and Wales (the “Company”), posted an updated investor presentation on its website. A copy of the Company’s presentation is furnished as Exhibit 99.1 to this Report on Form 6-K and is incorporated herein by reference.
The information in slide 23 of such presentation is hereby incorporated by reference into all effective registration statements filed by the Company under the Securities Act of 1933, as amended.
| Exhibit No. |
|
| 99.1 | Slide Presentation. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Akari Therapeutics, Plc | ||
| (Registrant) | ||
| By: | /s/ Rachelle Jacques | |
|
Name: |
Rachelle Jacques | |
| Title: | President and Chief Executive Officer | |
Date: February 15, 2023
Exhibit 99.1

Akari Therapeutics February 2023

2 Certain statements in this presentation constitute “forward - looking” statements within the meaning of Section 27A of the Securit ies Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward - looking statements reflect our current views about our plans, intentions, expectations, stra tegies an prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expect ati ons, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward - looking statements and will be affected by a variety of risks and factors that are beyond our control. Such risks and uncertainties for our company include, but are not limited to: needs for additional capital to fund o ur operations; our ability to continue as a going concern; uncertainties of cash flows and inability to meet working capital needs; an inability or delay in obtaining required regulato ry approvals for nomacopan (Coversin) and any other product candidates that may result in unexpected cost expenditures; our ability to successfully develop nomacopan as a treatment for COV ID - 19 related pneumonia and to successfully commercialize any product in that indication; our ability to obtain orphan drug designation in additional indications; risks inh erent in drug development in general, and risks specific to the development of potential treatments for COVID - 19 - related illnesses; uncertainties in obtaining successful clinical results f or nomacopan and any other product candidates and unexpected costs that may result from difficulties enrolling patients in our clinical trials; failure to realize any value of no macopan and any other product candidates developed or being developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; inabili ty to develop new product candidates and support existing product candidates; the approval by the FDA and EMA an any other similar foreign regulatory authorities of other competing or su perior products brought to market; risks resulting from unforeseen side effects; risk that the market opportunity for nomacopan may not be as large as expected; risks associated wit h t he impact of the outbreak of COVID - 19; inability to obtain, maintain and enforce patents another intellectual property rights or the unexpected costs associated with such enforc eme nt or litigation; inability to obtain and maintain commercial manufacturing arrangements with third party manufacturers or establish commercial scale manufacturing capabilities ; t he inability to timely source adequate supply of our active pharmaceutical ingredients from third - party manufacturers on whom the company depends; unexpected cost increases and pric ing pressures and risks and other risk factors detailed in our public filings with the U.S. Securities and Exchange Commission; including our most recently filed Annual Rep ort on Form 20 - F filed with the SEC. The statements made in this presentation speak only as of the date stated herein, and subsequent events and developments may cau se our expectations and beliefs to change. Unless otherwise required by applicable securities law, we do not intend, nor do we undertake any obligation to update or rev ise any forward - looking statements contained in this presentation to reflect subsequent information, events, results or circumstances or otherwise. While we may elect to update t hes e forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherw ise , except as required by law. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use shou ld not be construed as an endorsement of such products. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale o f t hese securities in any state or other jurisdiction. Forward - Looking Statements

Akari Overview 1 Novel bispecific recombinant protein targeting both complement C5 and leukotriene B4 (LTB4) 2 Broad therapeutic potential due to bispecific mechanism of action and multiple routes of administration (subcutaneous, topical, intravitreal, intravenous) Advanced late - stage program with extensive clinical and safety data 3 Lead indication in pediatric hematopoietic stem cell transplant - related thrombotic microangiopathy (HSCT - TMA) accelerated into pivotal Part B of Phase 3 clinical trial; granted Orphan and FDA Rare Pediatric Disease Designation (Priority Review Voucher eligible at approval) 4 5 Significant unmet need in both adult and pediatric HSCT - TMA with ~80% mortality rate and no approved therapies; added new pipeline program in adult HSCT - TMA, which will support both adult and pediatric regulatory pathways Pre - clinical program investigating long - acting PAS - nomacopan in geographic atrophy (GA) with potential to reduce sight - threatening choroidal neovascularization (CNV) risk via LTB4 inhibition and target dose interval beyond 3 months; IND submission first half of 2024 6 3

• Inhibits complement C5 activation similarly to eculizumab, but binds a different conserved region of C5 • Unique mode of action against leukotriene B4 (LTB4) tightly sequesters LTB4 within the protein ‘ligand capture’ preventing receptor - mediated cell activation • Nomacopan LTB4 inhibition is prolonged since nomacopan C5 complex has a half - life of about 60h and is present in excess to LTB4, so nomacopan circulates through the body and absorbs LTB4, disrupting cell recruitment and activation 4 High resolution structure of nomacopan (cyan) bound to the CUB, C5d, and C345C domains of C5 1 High resolution structure of nomacopan capture of LTB4 (yellow) )2 Nomacopan Is a Novel Bispecific Recombinant Protein References 1. Jore MM, Johnson S, Sheppard D, et al. Structural basis for therapeutic inhibition of complement C5. Nat Struct Mol Biol. 2016;23(5):378 - 386. 2. Roversi P, Ryffel B, Togbe D, et al. Bifunctional lipocalin ameliorates murine immune complex - induced acute lung injury. J Biol Chem. 2013;288(26):18789 - 18802. T icks modulate host responses to their own benefit • When ticks feed, they must control the host immune response, including inflammation, pain, itch and blood flow to avoid detection and successfully feed • They do this by secreting many immunomodulatory proteins in their saliva that have been perfected by evolution for potency, specificity and tolerability Novel, bispecific nomacopan Is derived from nature via discovery in ticks Two Modes of Action
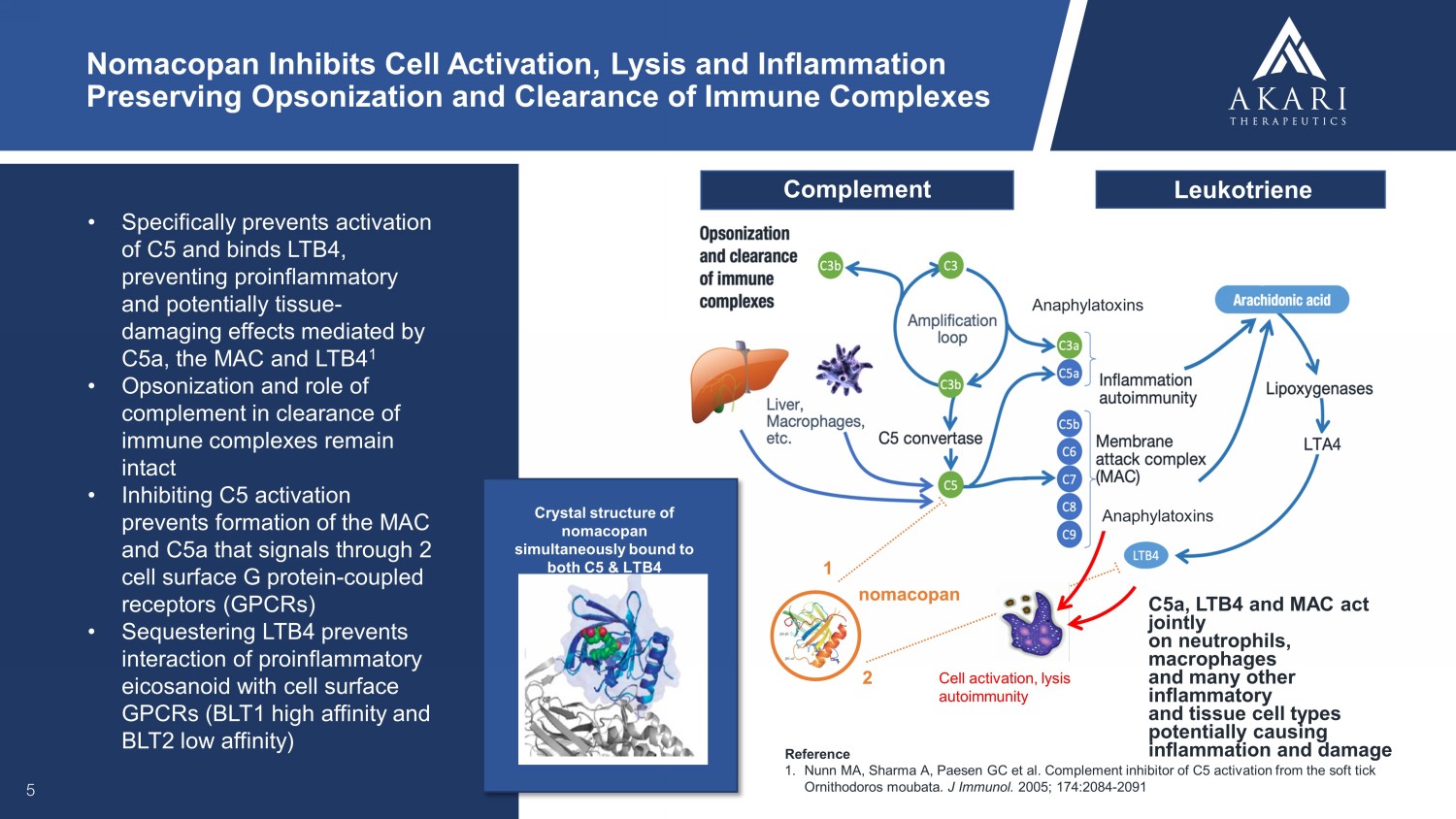
• Specifically prevents activation of C5 and binds LTB4, preventing proinflammatory and potentially tissue - damaging effects mediated by C5a, the MAC and LTB4 1 • Opsonization and role of complement in clearance of immune complexes remain intact • Inhibiting C5 activation prevents formation of the MAC and C5a that signals through 2 cell surface G protein - coupled receptors (GPCRs) • Sequestering LTB4 prevents interaction of proinflammatory eicosanoid with cell surface GPCRs (BLT1 high affinity and BLT2 low affinity) 5 Nomacopan Inhibits Cell Activation, Lysis and Inflammation Preserving Opsonization and Clearance of Immune Complexes Reference 1. Nunn MA, Sharma A, Paesen GC et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata . J Immunol. 2005; 174:2084 - 2091 Cell activation, lysis autoimmunity nomacopan 1 2 Complement Leukotriene Anaphylatoxins C5a, LTB4 and MAC act jointly on neutrophils, macrophages and many other inflammatory and tissue cell types potentially causing inflammation and damage Anaphylatoxins Crystal structure of nomacopan simultaneously bound to both C5 & LTB4

LTB4 & C5 Are Separate Pathways But In Vivo Data Point to Signalling Interplay That Leads to Damaging Inflammation References 1. Sadik CD, et al. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and Fc γ R signaling. Proc Natl Acad Sci U S A . 2012;109(46):E3177 - E3185. 2. Sadik CD et al. Lipid - cytokine - chemokine cascades o rchestrate leukocyte recruitment in inflammation . J Leuk Biol. 2012; 91(2 :207 - 215. ; 3. Roversi P, et al. Bifunctional lipocalin ameliorates murine immune complex - induced acute lung injury. J Biol Chem . 2013;288(26):18789 - 18802 • Neutrophils infiltrate joints by way of multiple chemoattractant receptors, including LTB4 (BLT1) and chemokine receptors • In the joint, neutrophils perpetuate their own recruitment by releasing LTB4 and IL - 1 β • Complement C5aR activation of neutrophils is required for LTB4 release and early neutrophil recruitment into the joint 6 • C5 and LTB4 contribute equally to this model of IC - ALI • C5a receptor signaling regulates Fc receptors promoting inflammation • Activated alveolar macrophages produce proteases, cytokines & LTB4 • C5a and LTB4 receptor activation upregulate adhesion molecules, recruit & degranulate neutrophils releasing super - oxides, causing further inflammation and microvascular damage LTB4 initiates cytokine and chemokine cascade in the joint Inflammation at the alveolar surface In vivo study of autoantibody - induced inflammatory arthritis 1, 2 In vivo study of immune complex - induced acute lung injury (IC - ALI) 3
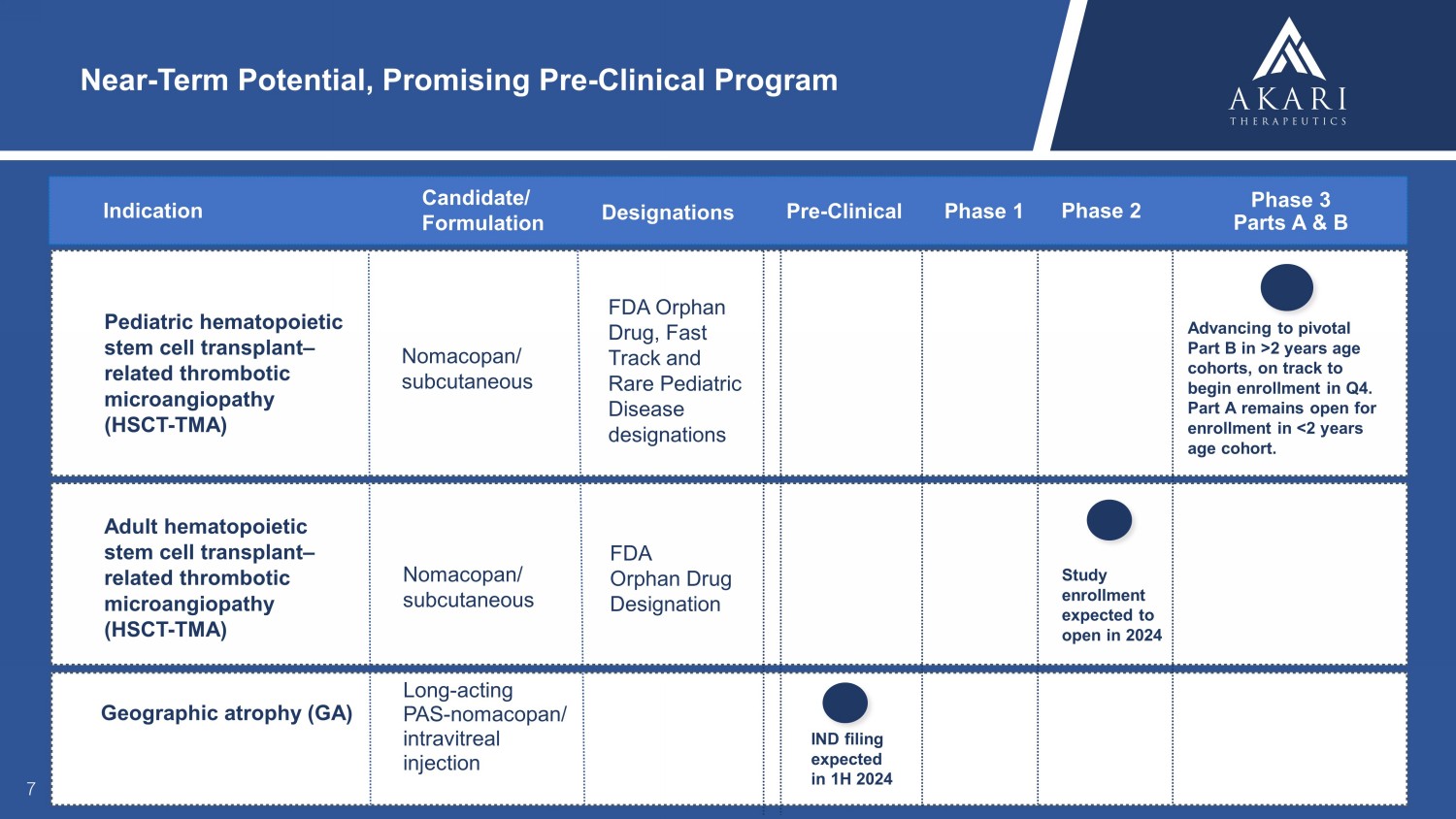
Geographic atrophy (GA) Long - acting PAS - nomacopan/ intravitreal injection FDA Orphan, Fast Track, and Rare Pediatric Disease designations Pediatric hematopoietic stem cell transplant – related thrombotic microangiopathy (HSCT - TMA) Nomacopan/ subcutaneous Near - Term Potential, Promising Pre - Clinical Program 7 FDA Orphan Drug, Fast Track and Rare Pediatric Disease designations Designations Indication Candidate/ Formulation Phase 3 Parts A & B Phase 1 Pre - Clinical Adult hematopoietic stem cell transplant – related thrombotic microangiopathy (HSCT - TMA) Nomacopan/ subcutaneous Phase 2 IND filing expected in 1H 2024 Study enrollment expected to open in 2024 Advancing to pivotal Part B in >2 years age cohorts, on track to begin enrollment in Q4. Part A remains open for enrollment in <2 years age cohort. FDA Orphan Drug Designation

• In addition to current areas of focus, Akari has conducted clinical research in several other areas, including Phase 2/3 clin ica l trials of subcutaneous nomacopan for treatment of bullous pemphigoid (BP) and paroxysmal nocturnal hemoglobinuria (PNH) • This research set a solid foundation for the current Phase 3 clinical trial in pediatric HSCT - TMA Previous Areas of Clinical Development, Including PNH and BP, Support Current Development Pathways • In clinical studies of nomacopan in BP, 7 of 9 patients responded to nomacopan 1 o 3 showed >80% reduction in BPDAI by day 42 (BP disease activity) All prior treatment, including steroids, withdrawn ~ one week prior to initiation of treatment with nomacopan. Lesional mometasone was administered to Day 21. • In a Phase 3 study in PNH, 100% of untreated patients were transfusion dependent while 0% of nomacopan patients were transfusion dependent o >32 patient years of nomacopan exposure in PNH in 19 patients Proportion of PNH patients who were transfusion independent following entry to trial 1. Sadik CD, et al. Evaluation of nomacopan for treatment of bullous pemphigoid a phase 2a non0randomized controlled trial. JAMA Dermatol. 2022; 158: 641 - 649 N=4 N=5 8

9 Discovered nomacopan Volution Torsten Hombeck, PhD Chief Financial Officer Miles Nunn, DPhil Chief Scientific Officer Rachelle Jacques President & CEO Melissa Bradford - Klug Chief Operating Officer John Neylan , MD Chief Medical Officer Leadership Team

10 THROMBOTIC MICROANGIOPATHIES (TMAs)

11 • TMA following a stem cell transplant procedure is a rare but serious complication of HSCT that appears to involve complement activation, inflammation, tissue hypoxia and blood clots, leading to progressive organ damage and death • Mortality in patients who develop severe transplant - related TMAs is 80% (across both adults and children) 1 • Currently, there are no approved treatment options in the U.S. or Europe Nomacopan May Be the First Treatment for Pediatric HSCT - TMA, a Condition with Mortality Up to 80% 1. Efficacy Efficacy of complement C5 inhibition by eculizumab is established in HSCT - TMA 2 and atypical hemolytic uremic syndrome ( aHUS ) 3,4 , another TMA (eculizumab); efficacy of nomacopan C5 inhibition supported by clinical PNH research 5 2. Dosing Nomacopan clinical trials are establishing a simple, fixed dose for each age category; rapid offset of action allows complement re - activation if/when needed 3. GVHD Graft versus host disease (GVHD) is commonly present in patients with severe HSCT - TMA 6; LTB4 is often elevated in patients with GVHD and inhibition of LTB4 may slow GVHD progression 7 1. Rosenthal J. Hematopoietic cell transplantation - associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, and treatment. J Blood Med. 2016;7:181 - 186. Published 2016 Sep 2 2. Jodele S, et al. Eculizumab therapy in children with severe hematopoietic stem cell transplantation - associated thrombotic microangiopathy. Biol Blood Marrow Transplant. 2014;20(4):518 - 525. 3. Licht C, et al. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2 - year extensions of phase 2 studies. Kidney Int. 2015;87(5):1061 - 1073. 4. Greenbaum LA, et al. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701 - 711. Kidney Int. 2016;90(3):709 5. Schols S, Nunn MA, Mackie I et al. Succesful treatment of a PNH patient non - responsive to eculizumab with novel complement C5 inhibitor covers (nomacopan). Br J Hematol . 2020; 188: 332 - 340. 6. Jodele S, et al. Complement blockade for TA - TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood . 2020;135(13):1049 - 1057. 7. Takatsuka H, et al. Predicting the severity of intestinal graft - versus - host disease from leukotriene B4 levels after bone marrow transplantation. Bone Marrow Transplant. 2000;26(12):1313 - 1316.
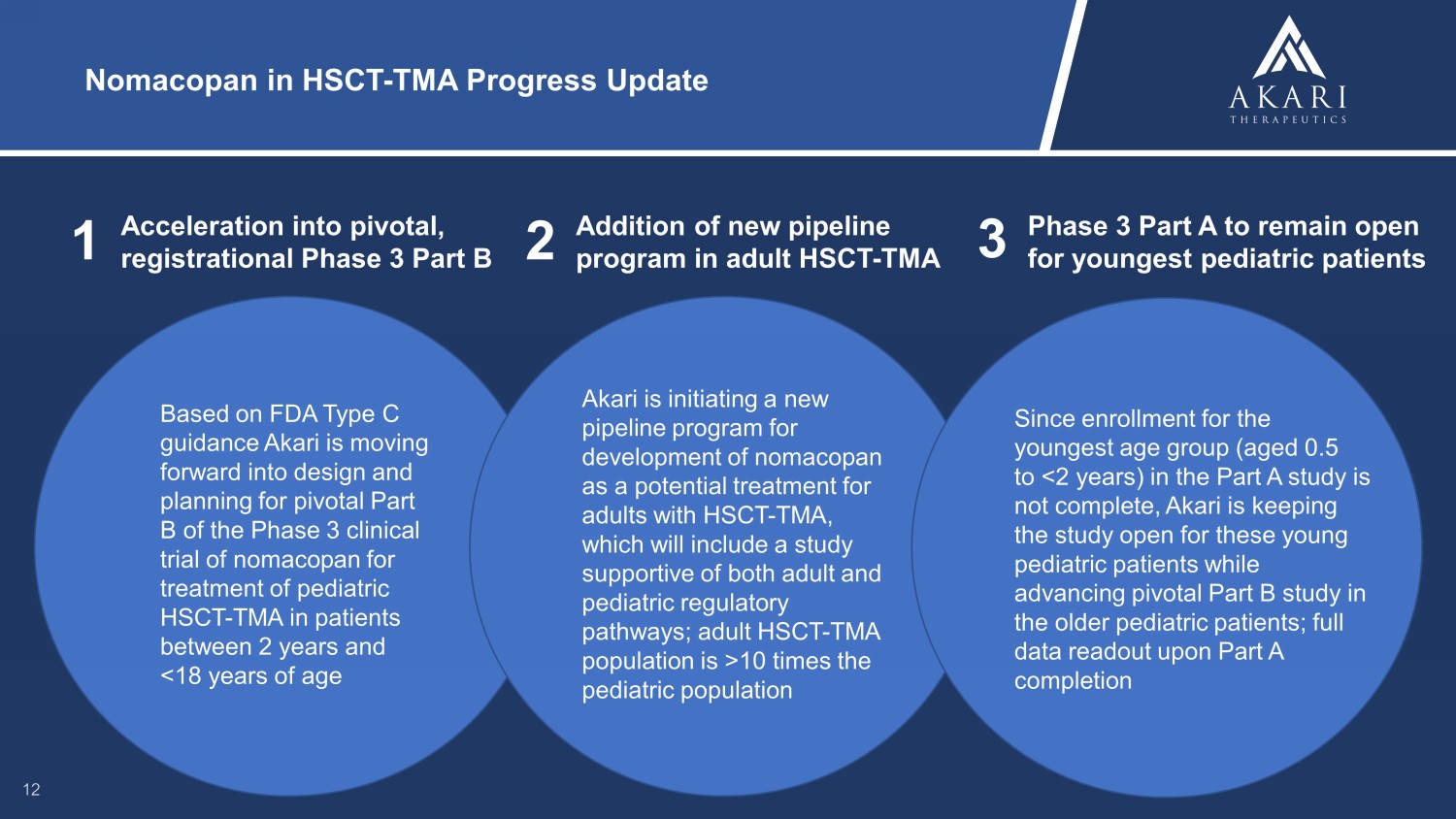
Nomacopan in HSCT - TMA Progress Update 1 Acceleration into pivotal, registrational Phase 3 Part B 12 Based on FDA Type C guidance Akari is moving forward into design and planning for pivotal Part B of the Phase 3 clinical trial of nomacopan for treatment of pediatric HSCT - TMA in patients between 2 years and <18 years of age 2 Addition of new pipeline program in adult HSCT - TMA 3 Phase 3 Part A to remain open for youngest pediatric patients Akari is initiating a new pipeline program for development of nomacopan as a potential treatment for adults with HSCT - TMA, which will include a study supportive of both adult and pediatric regulatory pathways; adult HSCT - TMA population is >10 times the pediatric population Since enrollment for the youngest age group (aged 0.5 to <2 years) in the Part A study is not complete, Akari is keeping the study open for these young pediatric patients while advancing pivotal Part B study in the older pediatric patients; full data readout upon Part A completion

References 1. FDA website. https://www.fda.gov/drugs/development - resources/model - informed - drug - development - pilot - program . Akari was selected to participate in the FDA Model - Informed Drug Development program that is helping accelerate development of treatments, including those for rare pediatric diseases Predictive PK/PD Model Akari’s robust, predictive PK/PD model simulated 10,000 virtual patients informing FDA MIDD interactions that helped confirm PK/PD model suitability and doses selected for Phase 3 Part A nomacopan study in severe pediatric HSCT - TMA MIDD Participation Akari clinical data from 38 subjects (in previous clinical studies and healthy volunteers) support PK/PD model simulations used to select doses for the nomacopan Phase 3 Part A clinical trial in HSCT - TMA Program Acceleration in Pediatric HSCT - TMA Rich Data Set 13 An expanded PK/PD model using data from 55 patients treated with nomacopan was reviewed in the recent Type C interaction with the FDA along with PK/PD data from Part A and found to be predictive supporting simple, fixed dosing in the upcoming Phase 3 pivotal Part B clinical trial

14 Part B Endpoint: TMA response including independence of RBC transfusion and/or urine protein creatinine ratio ≤ 2 mg/mg maintained ≥ 28 days immediately prior to any scheduled clinical visit up to Week 16 • Dose confirmation/safety • Sites in US, UK, Poland • Severe HSCT - TMA in 3 age cohorts o 0.5 to <2 years o ≥2 to <9 years o ≥9 to <18 years • Predictive PK/PD model compared with PK/PD data from patients enrolled in Part A in >2 years of age • <2 year age cohort not yet enrolled; Part A to stay open for these patients Phase 3 Part A Pediatric HSCT - TMA Phase 3 Advancing Into Pivotal Stage; Adult Phase 2 Supportive of Pediatric Program Phase 3 Clinical Trial in Pediatric HSCT - TMA Phase 2 Clinical Trial in Adult HSCT - TMA Phase 3 Part B NDA • Pivotal registrational study to support NDA for potential regulatory approval • Safety and efficacy • Two age cohorts o ≥2 to <9 years o ≥9 to <18 years • Study planning, design underway • Start of enrollment targeted for end of 2023 Data from Part A supports advanceme nt to pivotal Part B with simple, fixed dose for >2 years age cohorts Safety and efficacy data from Part B support a potential regulatory filing • Potential pediatric HSCT - TMA regulatory filing with FDA supported by data from pivotal pediatric Phase 3 Part B and supportive Phase 2 clinical trial in adult HSCT - TMA • Supports advancement into adult HSCT - TMA Phase 3 study • Adult Phase 2 and 3 clinical trials support potential adult HSCT - TMA NDA • Adult Phase 2 and pediatric Phase 3 support potential pediatric HSCT - TMA NDA

~ 4,600 ~ 400 ~ 3,400 0 2,500 5,000 7,500 10,000 Children: ~200 - 300 PATIENT POPULATION IN THOUSANDS HSCT - TMA U.S. Population (incidence in adults and children in the U.S. 2022) References • Health Resources and Services Administration (HRSA), 2020 • Jodele S, et al. Diagnostic and risk criteria for HSCT - associated thrombotic microangiopathy: a study in children and young adults. Blood. 2014;124(4):645 - 653. • Jodele S, et al. Complement blockade for TA - TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood. 2020;135(13):1049 - 1057. ~22,000 HSCTs occur annually in the U.S. ~8,400 (38%) lead to TMAs ~3,400 TMAs are moderate to severe complement - mediated o 200 - 300 pediatrics o 3,100 - 3,200 adult Moderate to severe not complement mediated Moderate to severe complement mediated Mild 15 Pediatric & Adult HSCT - TMA U.S. Market Opportunity Adult population >10X pediatric

16 GEOGRAPHIC ATROPHY (GA)

PAS - Nomacopan May Provide 3 Key Benefits: Complement Inhibition, Fewer Doses and LTB4 Inhibition to Address CNV Risk 17 • Long - acting PAS - nomacopan is being developed with a profile that has the potential to deliver efficacy benefits of complement inhibition with a fraction of the number of annual doses (compared with current late - stage complement - only inhibitors for GA) • Simultaneously, it may help address CNV safety risks reducing the total number of intravitreal injections (IVIs) needed to help preserve vision from GA as well as CNV References 1. Liao DS, et al., Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age - related macular degeneration - a randomised phase 2 trial. Opthalmology 2019; 127: 586 - 195. 2. Jaffe GJ, et al., C5 inhibitor avacincaptad peg for geographic atrophy due to age - related macular degeneration - a randomised pivotal phase 2/3 trial. Ophthalmology 2021; 128: 576 - 586. 3. McClard CK, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a patient - reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol . 2021;6(1):e000669. 4. Sasaki F, et al., Leukotriene B4 promotes neovascularisation and macrophage recruitment in murine wet - type AMD models. JCI Insight 2018; 3: e96902. 1. Efficacy Efficacy of complement C5 inhibition slowing progression of GA lesions is well understood 1,2 2. Frequency of Intravitreal Injections Less frequent needle injections into the back of the eye, a source of fear, discomfort and disruption for patients 3 ; p otential for only 3 to 4 injections with PAS - nomacopan each year 3. Safety LTB4 inhibition may prevent VEGF - A overexpression, a key driver of sight - threatening CNV, 4 a safety risk (treated with VEGF inhibitors) associated with current late - stage complement - only inhibitors 1,2 New PAS - nomacopan construct composition of matter patent filed Dec. 2022; if granted provides patent protection to 2042

PAS - Nomacopan in GA Progress Update 1 PAS - nomacopan development on track for IND submission 1H24 18 Based on progress, Akari is targeting an Investigational New Drug (IND) application submission to the U.S. Food and Drug Administration in the first half of 2024 2 New PAS - nomacopan versions fully active; target dosing interval extended beyond 3 months 3 New versions expressing well and on track toward GMP scale for clinical trials Pre - clinical development on long - acting PAS - nomacopan has successfully achieved multiple new and promising versions that are fully active, show high expression levels and have significantly larger hydrodynamic radii than earlier versions, which potentially increase the PAS - nomacopan target dosing interval beyond 3 months Development has progressed from lab scale to pre - GMP optimization and is on track to select the version of PAS - nomacopan that will advance to GMP manufacturing and non - clinical safety studies in preparation for an IND and the start of clinical trials in GA

19 References 1. Medscape article on 24 - month data presentation at AAO 2022 With Approval Pending, Pegcetacoplan Shows Mixed Results for Treating Geographic Atrophy https://www.medscape.com/viewarticle/981813#vp_2 2. Eyelea ® Prescribing Information https://www.regeneron.com/downloads/eylea_fpi.pdf 3. M c C la rd C K , e t al . Q ue s t ionnai re to A ss e s s Li f e I m pa c t o f T r eat m en t b y Int r avi t r ea l In j e c t ion s (Q UALI T II ). BM J O pe n O phtha l m ol . 2021;6 ( 1 ) :e000669. 4. Weston - Davies, W., et al. Development of long - acting PAS - nomacopan for treatment of GA and other retinal diseases. Poster presen tation ARVO, 2022. • Between monthly or every other month (EOM) treatment for GA and CNV, patients could face 18 injections a year 1,2 • Discontinuation for a late - stage complement - only GA treatment reported up to 20% 1 • For anti - VEGF CNV treatments, up to 1/3 of patients may discontinue/ not adhere 3 PAS - Nomacopan Has Potential to Address the Significant Patient Burden of Frequent Intravitreal Injections for GA/CNV Long - Acting PAS - Nomacopan Has Potential to Extend Intravitreal Dose Interval to 3 to 6 Months 4 Concentration of PAS - nomacopan in µg/mL of vitreous 0 10,000 1,000 100 25 50 75 100 125 150 175 10 1 Estimated minimal effective concentration for C5 ablation 0.1 PAS - 600 at 1 µg/mL Calculated line for PAS - 600 at 4 µg/mL Target for new PAS constructs under development Days

• CNV is an overdevelopment of blood vessels in the retina 2 • New blood vessels are leaky , fluid from blood/red blood cells enter the retina 2 • Fluid can distort/damage the retina , including photoreceptors 2 Neovascularization, fluid, retinal/macular distortion 2 CNV starts with inflammation in the choroid and retinal pigment epithelium (RPE) • The choroid is part of the vascular layer of the eye 1 • The RPE , adjacent to the choroid, is constantly exposed to high levels of metabolic and oxidative stress 1 choroid retinal pigment epithelium photoreceptor cells retina (macular) LTB4 Inhibition May Prevent Choroidal Neovascularization 1 • The RPEs ability to cope with stress decreases with age and the subsequent inflammation damages the RPE and photoreceptors 2 • Damaged RPE releases leukotrienes, including LTB4 2,3 LTB4 activation can lead to over expression of VEGF - A 2 • In a pre - clinical model of laser - induced CNV LTB4 recruited inflammatory immune cells into the retina 3 • M2 macrophages were attracted and activated via LTB4 receptors leading to production of vascular endothelial growth factor – A (VEGF - A) 3 3 Normal expression of VEGF - A is healthy • VEGF - A is one of the key factors responsible for endothelial cell proliferation and migration • Endothelial cells form the inner layer of blood vessels and play a key role in function, including exchanges between blood vessels and surrounding tissues 4 Overexpression of VEGF - A drives choroidal neovascularization LTB4 can upregulate the production of VEGF - A, a key driver of CNV. 3 CNV is responsible for 90% of severe vision loss in AMD patients and eyes with CNV experience greater vision loss than GA only VEGF - A protein References: 1. Hejtmancik JF, Nickerson JM. Overview of the Visual System. Prog Mol Biol Transl Sci. 2015;134:1 - 4. . 2. Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol . 2004;137(3):496 - 503. 3. Sasaki F, Koga T, Ohba M, et al. Leukotriene B4 promotes neovascularization and macrophage recruitment in murine wet - type AMD models. JCI Insight 2018;3(18):e96902. Published 2018 Sep 20. 4. Guyer D.R., et al. Subfoveal choroidal neovascular membranes in age - related macular degeneration. Visual prognosis in eyes with relatively good initial visu al acuity. Arch Ophthalmol . 1986;104:702 – 705 5. Wong T.Y., et al. The natural history and prognosis of neovascular age - related macular degeneration: A systematic review of the literature and meta - analysis. Ophthalmology 2008;115:116 – 126. 20

PAS - Nomacopan Decreased VEGF Levels As Effectively As An On - Market Anti - VEGF In a Pre - Clinical CNV Model V E G F ( p g / m l ) S a l i n e n o m a P A S - n o m a a n t i - V E G F H e a l t h y c t 0 100 200 300 p=0.044 p=0.048 Effect of PAS - nomacopan on VEGF levels in a standard pre - clinical model of severe uveitis References 1. Liao DS, et al., Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age - related macular degeneration - a randomised phase 2 trial. Opthalmology 2019; 127: 586 - 195. 2. Jaffe GJ, et al., C5 inhibitor avacincaptad peg for geographic atrophy due to age - related macular degeneration - a randomised pivotal phase 2/3 trial. Ophthalmology 2021; 128: 576 - 586. 3. Eskandarpour M, et al. Leukotriene B 4 and Its Receptor in Experimental Autoimmune Uveitis and in Human Retinal Tissues: Clinical Severity and LTB 4 Dependence of Retinal Th17 Cells. Am J Pathol . 2021;191(2):320 - 334. 4. Eskandarpour M, et al. Immune - Mediated Retinal Vasculitis in Posterior Uveitis and Experimental Models: The Leukotriene (LT)B4 - VEGF Axis. Cells 2021;10(2):396. Published 2021 Feb 15. 21 • PAS - nomacopan is a bispecific inhibitor of LTB4 and complement (C5) o Complement inhibition has shown efficacy in slowing progression of GA 1 - 3 o LTB4 inhibition may also reduce the risk of sight - threatening choroidal neovascularization (CNV), a safety risk associated with clinical trials of current late - stage complement - only inhibitors 1 - 3 • In a standard pre - clinical model of laser - induced CNV, long - acting PAS - nomacopan (single IVI) decreased VEGF levels as effectively as anti - VEGF treatment Eylea ® (multiple IVIs) 1,2 • Positive results from the pre - clinical studies 3,4 support the advancement of long - acting PAS - nomacopan toward IND/IMPD for intravitreous injection (IVI) and clinical trials in GA as well as the potential for the investigational treatment to address areas of unmet patient needs, including risk of CNV Intravitreal VEGF concentration ( pg /ml) Nomacopa n Saline PAS - Nomacop an Anti - VEGF Healthy control

22 FINANCIAL UPDATE

Financial Update 23 • Ticker: AKTX (NASDAQ) • 74.4M ADS outstanding • Cash of $16M as of September 30, 2022 • Estimated cash runway into Q3 2023

24 THANK YOU
