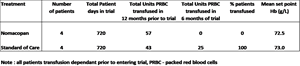Akari Therapeutics Announces Positive Interim Data from Phase III Paroxysmal Nocturnal Hemoglobinuria (PNH) Study
- Phase III interim data on the first eight PNH patients who were all transfusion dependent at entry to the CAPSTONE trial show that all four patients randomized to nomacopan were transfusion independent for the first six months of treatment while all four patients on standard of care (SOC) remained transfusion dependent
- Fourteen PNH patients across four clinical trials have now been treated with nomacopan for more than 25 cumulative patient-years, with data showing that self-administration is well tolerated with no reported drug-related serious adverse events
- Akari has orphan status from the
U.S. Food and Drug Administration (FDA) andEuropean Medicines Agency (EMA) for nomacopan for treatment of PNH
NEW YORK and LONDON,
|
|||||
Dr Austin Kulasekararaj of King’s
PNH is an orphan hematological condition that, if left untreated, results in a high mortality rate. Eculizumab, a C5 complement inhibitor, is approved for the treatment of the disease. Nomacopan is also a C5 complement inhibitor which also binds the proinflammatory eicosanoid leukotriene B4 (LTB4).
In the Phase III CAPSTONE trial, PNH patients, naïve to anti-complement treatment, are randomized to receive daily subcutaneous nomacopan or SOC (blood transfusion with or without anticoagulation) for six months. After six months, patients on SOC receive nomacopan for three months and patients on nomacopan continue the drug for a further three months. The trial design was agreed with the FDA following an end of Phase II meeting. The primary endpoint of this study is: haemoglobin (Hb) stabilization defined as Hb greater than the set point for each patient set during the pre-study randomization period, and the avoidance of packed red blood cell transfusions during the treatment period of 180 days. All patients receive a transfusion if they fall below the Hb set point.
The interim data tabulated below shows that the four patients on nomacopan met the primary endpoint and received no transfusions for the first six months of treatment while all four patients on placebo were transfused during the first six months of treatment. Both groups had similar historic transfusion levels and mean Hb set points. The difference is statistically significant at the p=0.034 level.
In the Phase II COBALT PNH study six out of eight patients were transfusion dependent prior to treatment. Three became transfusion independent during the three-month Phase II study and the remaining three became transfusion independent during treatment with nomacopan in the long-term follow-up safety study, where transfusion independence is defined as no transfusion for 6 months. Of these six patients all have now continually maintained transfusion independence for up to two years except one patient who has received one unit of PRBC in the last 12 months.
In total, 14 PNH patients have been treated with nomacopan across four trials: Phase II, Phase III, PNH eculizumab resistant study, and PNH long-term safety and efficacy study. Additional interim Phase III data is expected to be announced during the European Hematology Association Annual Congress being held
Akari is currently developing a new higher concentration formulation of nomacopan allowing a small volume 0.3mL, low viscosity injection, with an insulin pen-like injector holding one week’s daily dosing stable at room temperature, improving both patient comfort and convenience. Recruitment into the Phase III CAPSTONE study is currently deferred in order to potentially take advantage of this new formulation.
“This interim PNH transfusion further demonstrates the positive patient treatment benefit and tolerability of nomacopan in PNH patients,” commented Clive Richardson, CEO of Akari Therapeutics. “The positive PNH data in turn supports our lead programs in bullous pemphigoid (BP), pediatric hematopoietic stem cell transplant-related thrombotic microangiopathy (HSCT-TMA) and atopic keratoconjunctivitis (AKC), where C5 and LTB4 may both play a role in disease pathology.”
About Akari Therapeutics
Akari is a biopharmaceutical company focused on developing inhibitors of acute and chronic inflammation, specifically for the treatment of rare and orphan diseases, in particular those where the complement (C5) or leukotriene (LTB4) systems, or both complement and leukotrienes together, play a primary role in disease progression. Akari's lead drug candidate, nomacopan (formerly known as Coversin), is a C5 complement inhibitor that also independently and specifically inhibits leukotriene B4 (LTB4). Nomacopan is currently being clinically evaluated in four indications: bullous pemphigoid (BP), atopic keratoconjunctivitis (AKC), thrombotic microangiopathy (TMA), and paroxysmal nocturnal hemoglobinuria (PNH). Akari believes that the dual action of nomacopan on both C5 and LTB4 may be beneficial in AKC and BP. Akari is also developing other tick derived proteins, including longer acting versions.
Cautionary Note Regarding Forward-Looking Statements
Certain statements in this press release constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements related to the offering, the expected gross proceeds and the expected closing of the offering. These forward-looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control. Such risks and uncertainties for our company include, but are not limited to: needs for additional capital to fund our operations, our ability to continue as a going concern; uncertainties of cash flows and inability to meet working capital needs; an inability or delay in obtaining required regulatory approvals for nomacopan and any other product candidates, which may result in unexpected cost expenditures; our ability to obtain orphan drug designation in additional indications; risks inherent in drug development in general; uncertainties in obtaining successful clinical results for nomacopan and any other product candidates and unexpected costs that may result therefrom; our ability to enter into collaborative, licensing, and other commercial relationships and on terms commercially reasonable to us; difficulties enrolling patients in our clinical trials; failure to realize any value of nomacopan and any other product candidates developed and being developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; inability to develop new product candidates and support existing product candidates; the approval by the FDA and EMA and any other similar foreign regulatory authorities of other competing or superior products brought to market; risks resulting from unforeseen side effects; risk that the market for nomacopan may not be as large as expected; risks associated with the departure of our former Chief Executive Officers and other executive officers; risks associated with the SEC investigation; inability to obtain, maintain and enforce patents and other intellectual property rights or the unexpected costs associated with such enforcement or litigation; inability to obtain and maintain commercial manufacturing arrangements with third party manufacturers or establish commercial scale manufacturing capabilities; the inability to timely source adequate supply of our active pharmaceutical ingredients from third party manufacturers on whom the company depends; unexpected cost increases and pricing pressures and risks and other risk factors detailed in our public filings with the
For more information
Investor Contact:
Peter Vozzo
Westwicke
(443) 213-0505
peter.vozzo@westwicke.com
Media Contact:
Sukaina Virji / Nicholas Brown / Lizzie Seeley
+44 (0)20 3709 5700
Akari@consilium-comms.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/3683d2d3-314a-4a99-89c1-bc186f9ff0af
Source: Akari Therapeutics Plc