Akari Therapeutics Announces New Data from Atopic Keratoconjunctivitis (AKC) Patients Supporting the Potentially Beneficial Role of Nomacopan as an Inhibitor of both C5 and LTB4 in the Ongoing Phase I/II AKC Trial
- Biopsy tissue expresses the complement C5a receptor 1 and the leukotriene LTB4 receptor BLT1 on and within the surface of the eye, a previously unreported finding
- These new findings suggest that inhibition of both LTB4 and C5 by nomacopan may provide therapeutic effect by reducing both proinflammatory pathways, and redness, mucous and excess tearing associated with activation of BLT1 and C5a receptors
- Nomacopan can be delivered comfortably as eye drops to the surface of the eye, thereby potentially inhibiting C5 and LTB4 and preventing activation of the BLT1 and C5a receptors
- These new findings for the role of C5 and LTB4 in the surface of the eye support the positive efficacy signals seen in Part A of the Phase I/II trial in AKC patients treated with nomacopan.
NEW YORK and LONDON,
|
|||||
Immunofluorescence imaging of conjunctival tissue taken from AKC patients by Professor Virginia Calder and her team at the
Professor Calder said, “These findings are significant and tie in well with our previous work showing that nomacopan, which blocks both complement C5 and LTB4 activity, is likely to have a strong anti-inflammatory effect and be more effective than other treatment options given the heterogenous inflammatory environment in the eye.”
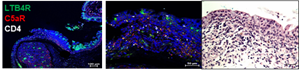
The images linked below show that the C5aR1 receptor (staining red) and the BLT1 receptor (staining green) are separately located in a section taken from an inflamed conjunctival papilla at X200 (A) and X400 (B) magnification. Section B shows that CD4 +T cells (white) are in close proximity to both receptors, which provides a potential link to the decrease in CD4 positive T cells seen in mice with experimental allergic conjunctivitis treated with nomacopan eye drops. Section C shows conventional hematoxylin and eosin (HE) stained section from same papilla for morphologic orientation (nuclei are purple and cytoplasm/extracellular matrix are pink).
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/b916b775-96ba-4e64-9099-bf9d99176f41
Recruitment into Part B of TRACKER, Akari’s Phase I/II clinical trial of topical nomacopan in AKC is ongoing and an interim data readout is expected at the end 2019. This follows on from completion of Part A of TRACKER which confirmed the safety and comfort of the drops in this first-in-eye study, but also saw within 2 months a strong efficacy signal with a 55% mean improvement in total clinical score (comprising signs and symptoms) in severe AKC patients receiving nomacopan in addition to standard of care cyclosporin. Cyclosporin is the standard of care treatment and Patients in Part A had received cyclosporin for at least three months prior to treatment with nomacopan.
Clive Richardson, Chief Executive Officer of Akari, said, “This new data provides a strong scientific rationale for our approach of dual targeting the complement and leukotriene systems in this case in combating epithelial inflammation in the eye, the benefit of which is evidenced in our promising clinical response data from Part A of the AKC study. The dual functionality of nomacopan has also been shown in several other diseases including bullous pemphigoid, rheumatoid arthritis and immune-complex lung disease demonstrating the broad potential of this unique treatment approach.”
About Akari Therapeutics
Akari is a biopharmaceutical company focused on developing inhibitors of acute and chronic inflammation, specifically for the treatment of rare and orphan diseases, in particular those where the complement (C5) or leukotriene (LTB4) systems, or both complement and leukotrienes together, play a primary role in disease progression. Akari's lead drug candidate, Nomacopan (Coversin), is a C5 complement inhibitor that also independently and specifically inhibits leukotriene B4 (LTB4) activity. Nomacopan (Coversin) is currently being clinically evaluated in four indications: bullous pemphigoid (BP), atopic keratoconjunctivitis (AKC), thrombotic microangiopathy, or TMA, and paroxysmal nocturnal hemoglobinuria (PNH). Akari believes that the dual action of Nomacopan (Coversin) on both C5 and LTB4 may be beneficial in AKC and BP. Akari is also developing other tick derived proteins, including longer acting versions.
Cautionary Note Regarding Forward-Looking Statements
Certain statements in this press release constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a variety of risks and factors that are beyond our control. Such risks and uncertainties for our company include, but are not limited to: needs for additional capital to fund our operations, our ability to continue as a going concern; uncertainties of cash flows and inability to meet working capital needs; an inability or delay in obtaining required regulatory approvals for Nomacopan (Coversin) and any other product candidates, which may result in unexpected cost expenditures; our ability to obtain orphan drug designation in additional indications; risks inherent in drug development in general; uncertainties in obtaining successful clinical results for Nomacopan (Coversin) and any other product candidates and unexpected costs that may result therefrom; difficulties enrolling patients in our clinical trials; failure to realize any value of Nomacopan (Coversin) and any other product candidates developed and being developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; inability to develop new product candidates and support existing product candidates; the approval by the FDA and EMA and any other similar foreign regulatory authorities of other competing or superior products brought to market; risks resulting from unforeseen side effects; risk that the market for Nomacopan (Coversin) may not be as large as expected; risks associated with the departure of our former Chief Executive Officers and other executive officers; risks related to material weaknesses in our internal controls over financial reporting and risks relating to the ineffectiveness of our disclosure controls and procedures; risks associated with the SEC investigation; inability to obtain, maintain and enforce patents and other intellectual property rights or the unexpected costs associated with such enforcement or litigation; inability to obtain and maintain commercial manufacturing arrangements with third party manufacturers or establish commercial scale manufacturing capabilities; the inability to timely source adequate supply of our active pharmaceutical ingredients from third party manufacturers on whom the company depends; unexpected cost increases and pricing pressures and risks and other risk factors detailed in our public filings with the
For more information
Investor Contact:
Peter Vozzo
(443) 213-0505
peter.vozzo@westwicke.com
Media Contact:
Mary-Jane Elliott / Sukaina Virji / Nicholas Brown
+44 (0)20 3709 5700
Akari@consilium-comms.com
Source: Akari Therapeutics Plc